release time:2021-07-26 15:11:59
Common in vitro diagnostic devices:
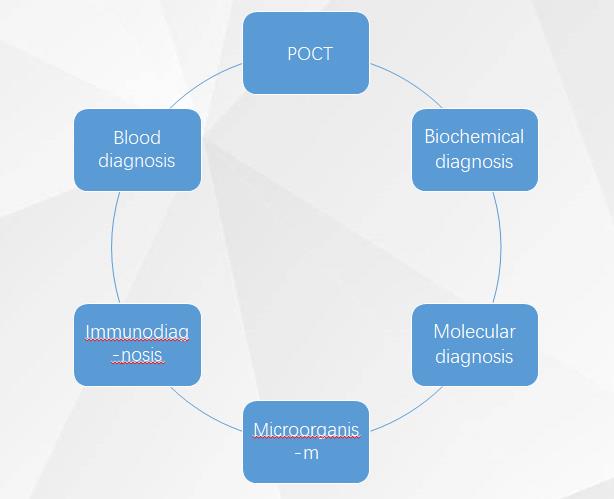
According to the detection category, it can be divided into blood test, biochemical diagnosis, immunodiagnosis, molecular diagnosis, microbiological diagnosis, POCT, etc.
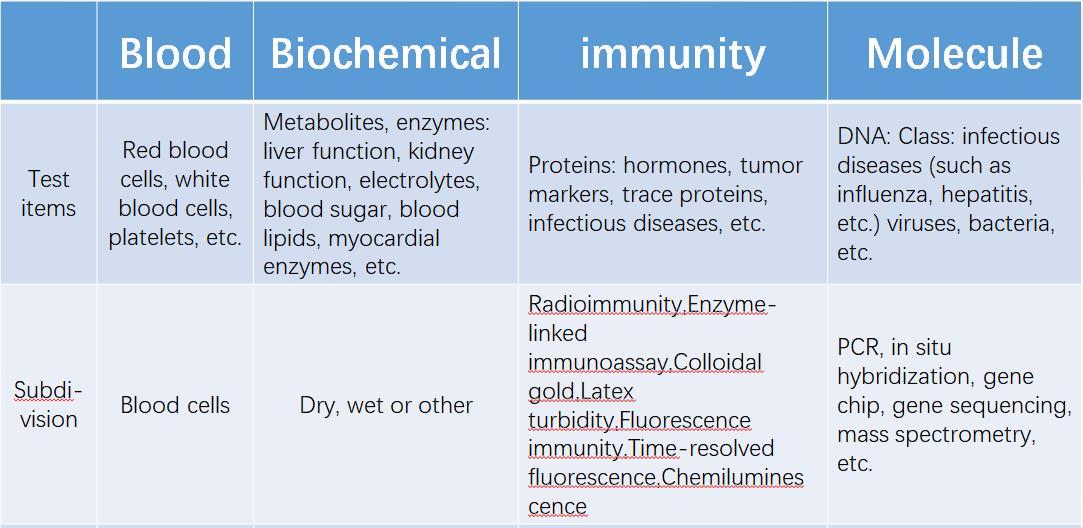
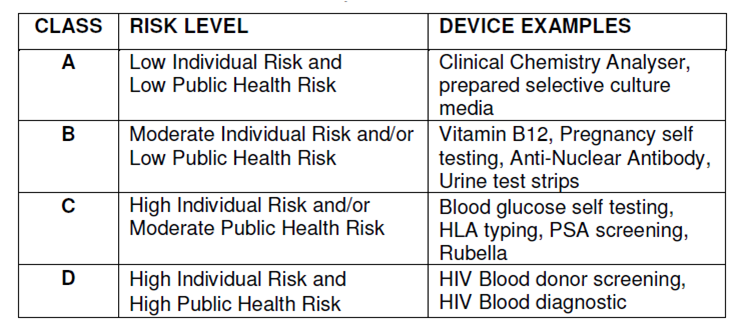
The FDA classifies medical devices (including IVD products) into Class I, Class II, or Class III based on the level of regulatory control required to reasonably ensure safety and effectiveness. The classification of IVD (or other medical devices) determines the appropriate pre-marketing process.
2022-03-07
The main reason why hyperlipidemic specimens interfere with biochemical tests is that the celiac particles in lipid blood cause lipid clouding.

2021-10-13
Chemiluminescent immunoassay, as an important technical tool for in vitro diagnostic testing technology, emerged in the mid-1970s. Nowadays, chemiluminescence immunoassay has become a mature and advanced technique for the detection of ultra-micro-active substances. It has a wide range of applications.

2021-09-16
The dry chemistry analyzer consists of highly sensitive multi-layer reagent coated slides. It is used in combination with dry chemistry reagents. The dry chemistry analyzer can be used for rapid testing of clinical biochemical items. Only 10 ml to 50 ml of sample is required. The results of a dry chemistry analyzer are comparable to those of a traditional wet chemistry analyzer.