release time:2024-06-12 15:28:53
LU Ying-chun1*, RAN Peng2* , ZHANG Xiao-yong1* , ZOU Liang-hong3* , WANG Bin2*
(1. Laboratory Department of Jinan Dean medical laboratory center, Jinan Shandong 250098; 2. Chengdu Seamaty Technology Co., Ltd., Chengdu Sichuan 611730; 3. Chengdu Pulitai Biotechnology Co., Ltd., Chengdu Sichuan 611730)
ABSTRACT: Objective/ To explore the comparative analysis of cholinesterase detected by seamaty SD1 automatic dry biochemical analyzer and Roche Cobas 6000 c501 automatic biochemical analyzer. To provide reference for the establishment of a simple and reliable cholinesterase determination method. Methods/ The samples of cholinesterase tested in our laboratory from April to June 2021 were selected as research objects. The precision and linear range of the two detection systems were evaluated, and then the methods were compared and the bias was evaluated respectively. Results/ The precision of CHE measured by the two detection systems were less than 3%. All the linearities meet the requirements. The method comparison and bias verification of the two detection systems are successful. Conclusion/ The precision and linearity of the two detection systems are good, and the detection results are consistent, which is worthy of popularization and application.
{This paper has been published in the World Latest Medicine Information Digest, Volume 22, Issue 20, 2022.}
DOI:10.3969/j.issn.1671-3141.2022.020.013
In recent years, with the continuous advancement of modern medical technology, the level of laboratory medicine has significantly improved. To meet various testing needs, it is common practice for laboratories to use two or more different analytical systems to measure the same item. However, discrepancies in results can occur when the same item is tested on the same sample using different systems. Ensuring the consistency of these results has become a focal point of discussion and concern within the testing community. For medical laboratories, achieving comparable results for the same test item across different systems is the ultimate goal of quality management .
Cholinesterase, an enzyme that catalyzes the hydrolysis of acylcholines, is also known as acylcholine hydrolase. Generally, cholinesterase can be classified into two types: (1) butyrylcholinesterase (CHE), often referred to as pseudocholinesterase, and (2) acetylcholinesterase (ACHE), known as true cholinesterase. Various methods are currently available to measure CHE, each with its own advantages and disadvantages. The reference values and ranges can vary depending on the method, making the choice of an appropriate method particularly important. This study explores the clinical value of measuring cholinesterase using an automatic dry biochemical analyzer, as reported below.
Quality Control Products: Randox Biochemical Control Products (1380UN, 1070UE).
Clinical Samples: Cholinesterase samples tested in our laboratory from April 2021 to June 2021 were selected for this study.
Instruments: Seamaty SD1 Automatic Dry Biochemical Analyzer (Chengdu Seamaty Technology Co., Ltd., hereafter referred to as Seamaty), cobas 6000 c501 Automatic Biochemical Analyzer (Roche).
Reagents: Comprehensive III Biochemical Assay Kit (Microfluidic Dry Chemistry Method) (Seamaty) and CHE (Butyrylthiocholine Substrate Method) Kit (Roche).
1.3.1 Precision Evaluation
Precision was validated for both detection systems using CLSI EP15 guidelines. Each day, a batch of experiments was conducted using samples of at least two concentration levels, with each sample measured five times over five days. The samples were selected from Randox biochemical control products (1380UN, 1070UE).
1.3.2 Linearity Range Evaluation
The linearity range of both detection systems was validated using CLSI EP06 guidelines. Samples with concentrations close to the high end of the linear range were mixed with low-concentration samples to create at least five different concentration gradients (xi). Each concentration was tested at least twice using both detection systems, and the average value (yi) was used to calculate the linear regression equation and the correlation coefficient (r).
1.3.3 Method Comparison and Bias Assessment
At least 40 patient samples were analyzed over a minimum of five working days. Each patient sample was measured twice using both the test and reference methods. Results were analyzed for consistency within each batch, ensuring that at least 50% of the sample results were outside the laboratory’s reference range. Data were analyzed using EP Evaluator software.
Using the two detection systems to measure Randox biochemical control products, the coefficient of variation (CV) for low and high values within batches were SD1 (1.39%, 1.25%) and c501 (1.52%, 1.40%). The automatic dry biochemical analyzer demonstrated better precision compared to the automatic biochemical analyzer (Table 1).
2.2 Linearity Evaluation
Comparison of the CHE precision for the two instruments
Unit: U/L
SD1
c501
1380UN
1070UE
1380UN
1070UE
Mean value
5627
11196
5651
11194
Variance
82
152
84
151
Coefficient of variation
1.39%
1.25%
1.52%
1.40%
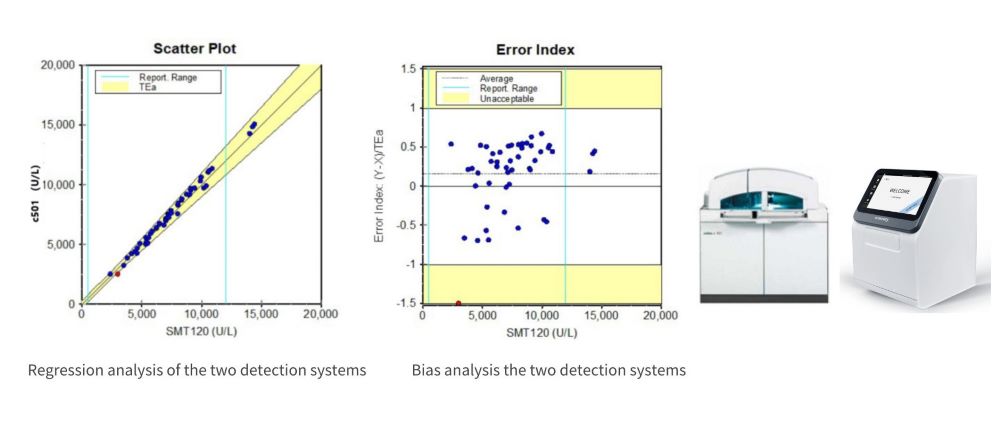
Setting each concentration’s set value as X and the measured value as Y, linear regression showed correlation coefficients of 0.9999 and 0.9998 for the CHE results from the two biochemical analysis systems, indicating good linear regression and high stability, not easily affected by random errors (Table 2).
2.3 Acceptability Evaluation of Different Detection Systems
Comparison of the linear range of the two detection systems
Detecting system
Linear interval
Regression equation
r
SD1
500-12000U/L
y=0.9976x - 10.953
0.9999
c501
500-12000U/L
y=1.0013x - 91.784
0.9998
This study used the Roche cobas 6000 c501 as the target detection system (Y) and the Seamaty SD1 as the system under evaluation (X). Sample concentrations covered the detection range, and the regression equation of cholinesterase measurement results was used as the basis. The relative and absolute biases between the two sets of data were compared, showing that the two instruments had small biases and good correlation.
Cholinesterase (CHE) exists in various forms in the body and can be classified into pseudocholinesterase and true cholinesterase. Pseudocholinesterase is widely distributed in the intestines, kidneys, liver, plasma, and glial cells, with low specificity to Ach and can hydrolyze other cholin esters such as succinylcholine. True cholinesterase is primarily found in cholinergic nerve terminal synaptic gaps and in red blood cells and cholinergic neurons .
Measuring serum cholinesterase activity is an effective method for diagnosing liver cell damage and organophosphorus poisoning. Ensuring the accuracy and repeatability of test results is crucial. Clinical laboratories often use different detection systems at different times, leading to variations in results due to different methods and lack of unified reference ranges. Therefore, comparing different methodologies is essential. Before conducting clinical trials, the precision of various systems should be analyzed to determine if they meet clinical requirements.
If the comparison meets clinical requirements and the results are comparable, calibration is unnecessary, and the results can be directly used in clinical practice. If there are discrepancies, close attention to quality control results is necessary.
This study found that the indicators of the Roche detection system and the Seamaty SD1 detection system were within acceptable ranges, indicating a certain degree of comparability. The Seamaty SD1 detection system demonstrated high clinical diagnostic and treatment value.
The Kubelka-Munk theory underpins dry chemistry, where reflectance (R) is related to factors such as the scattering coefficient (S) of the solid phase reaction layer, the light absorption coefficient per unit thickness (K), and the thickness of the solid phase layer (X). With S and X fixed, R is only related to K, and the concentration of the analyte is proportional to K. Reflectometry measures the R value to calculate the concentration of the analyte.
The Seamaty SD1 detection mechanism involves cholinesterase catalyzing the hydrolysis of acetylthiocholine at 37°C, producing thiocholine. Thiocholine reacts with yellow ferricyanide [Fe(CN)6]3-, forming colorless [Fe(CN)6]4-. The decrease in absorbance at 405 nm is proportional to cholinesterase activity. The analyzer calculates the activity (U/L) using stored formulas.
The colorimetric method used by Roche involves cholinesterase catalyzing the hydrolysis of butyrylthiocholine into thiocholine and butyrate. Thiocholine reduces yellow ferricyanide (III) to colorless ferrocyanide (II), with the color change measurable by colorimetry.
Studies show that while the butyrylthiocholine method for CHE measurement is reliable and accurate, it has drawbacks. The color reagent 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB) is unstable, prone to spontaneous decomposition, and has a short shelf life after opening, affecting result accuracy if not used promptly . This method is suitable for high-volume laboratories with timely result issuance requirements.
The Seamaty SD1's test strips are individually packaged for immediate use, easy to store, and highly stable, making them suitable for small-volume laboratories without centralized testing needs. This study found that both instruments had CVs <3%, demonstrating good repeatability. Compared to the Roche cobas 6000 c501, the Seamaty SD1's precision meets industry standards, making it suitable for clinical testing alongside other systems.
Generally, cholinesterase (pseudocholinesterase, cholinesterase II) is present in the liver, pancreas, heart, serum, and brain white matter. Serum cholinesterase should not be confused with erythrocyte acetylcholinesterase (EC 3.1.1.7), also known as cholinesterase I. The biological function of cholinesterase is unclear. Clinically, serum cholinesterase indicates organophosphorus pesticide poisoning and liver function. Preoperative screening for cholinesterase can detect atypical cholinesterase, avoiding prolonged respiratory arrest from muscle relaxants.
CHE measurement is significant for its decreased activity, indicating liver dysfunction. Studies report that CHE activity decline is an effective clinical diagnosis and emergency response indicator in organophosphorus poisoning patients.
In emergency work, due to the infrequency of organophosphorus poisoning cases, the stability of reagents can decrease when few samples are tested after opening a box of cholinesterase reagents. Dry chemistry detection of CHE offers unique packaging benefits, ensuring better stability and result accuracy, and is suitable for clinical needs with high application value. Literature also reports good correlation between dry chemistry test paper methods and automatic biochemical analyzers for amylase, urea, creatinine, and glucose measurements.
This study found good correlation between the two instruments for CHE measurement, with the Seamaty SD1's individually packaged test strips offering high stability and mitigating the instability issues of automatic biochemical analyzer reagents. This enhances result accuracy and flexibility in the laboratory, ensuring reliability.
In conclusion, the Seamaty SD1 automatic dry biochemical analyzer demonstrates excellent accuracy and precision for cholinesterase measurement, particularly beneficial for diagnosing and treating organophosphorus poisoning. Its simplicity, high precision, reliable results, and stable, easy-to-store reagents meet clinical emergency medicine requirements, making it highly valuable for widespread use.
[1]. Zhang, Yingying, and Tao Qingsong. "Comparison and Bias Assessment of 15 Routine Biochemical Test Results Across Different Detection Systems." Clinical Medicine and Laboratory Medicine 8, no. 3 (2011): 257-259.
[2]. Dündar, Muhammed, Karcı Hüseyin, Özdemir İlknur, et al. "Corrigendum to ‘New Silver N-Heterocyclic Carbenes Complexes: Synthesis, Molecular Docking Study and Biological Activities Evaluation as Cholinesterase Inhibitors and Antimicrobials’ [Journal of Molecular Structure 1238 (2021) 130399]." Journal of Molecular Structure 1241 (2021).
[3]. Zhang, Guichun. "Comparison of Homocysteine Detection Methods Using MP1 Dry Analyzer and Automatic Biochemical Analyzer." China Health Industry 15, no. 23 (2018): 176-179.
[4]. Wang, Shaoqin, Wang Wenjuan, and Yang Xiulan. "Comparison and Bias Analysis of Dry and Wet Chemical Detection Results for Some Emergency Biochemical Projects." Laboratory Medicine and Clinic (Supplement 2) (2014): 99-101.
[5]. Xu, Chuanhua, Yuan Shimei, Liao Liya, et al. "Application of Biological Variation Quality Standards in the Comparison Between Dry and Conventional Chemical Detection Systems." Modern Medicine and Health 36, no. 23 (2020): 3780-3782.
[6]. Wei, Jianwei. "Comparative Analysis of Lactate Dehydrogenase Measurement Results in Dry and Wet Chemical Detection Systems." Laboratory Medicine and Clinic 7, no. 12 (2010): 1231-1232.
[7]. Shi, Guanghua, Zhang Qing, Liu Lianyi, et al. "Application of Biological Variation Quality Standards in Fully Automatic Dry Biochemical Analyzers." Immunoassay and Clinical Analysis 24, no. 12 (2017): 1432-1436.
[8]. Ma, Chunhong, and Zhao Chunyan. "Evaluation of the Linearity of Amylase Detection Using MP1 Dry Biochemical Analyzer with EP6-A Document." Laboratory Medicine and Clinic 13, no. 18 (2016): 2681-2682.
[9]. Cai, Diya, Yang Ying, Wang Rui, et al. "Routine Maintenance and Troubleshooting of the DRI-CHEM7000 Fully Automatic Dry Biochemical Analyzer." Laboratory Medicine and Clinic 10, no. 11 (2013): 1485.
[10]. Ma, Yong, and Cui Han. "Clinical Significance of Cholinesterase Activity Detection in the Diagnosis and Treatment of Organophosphorus Poisoning." Medical Review 23, no. 2 (2017): 372-374.
[11]. Gong, Likun. "Correlation Analysis of Biochemical Item Detection Between Dry Chemistry Analyzers and Wet Chemistry Analyzers." Laboratory Medicine and Clinic 10, no. 4 (2013): 481-482.

2024-06-25
Discover Seamaty's VQ1 Fully Automated PCR Instrument, a breakthrough in pet diagnostics. Offering rapid, precise, and safe testing, it revolutionizes early detection and treatment of infectious diseases in pets. Ideal for veterinary hospitals and breeding centers, ensuring comprehensive and accurate health solutions for pets.

2021-12-06
As we all know, biochemical tests are one of the common items for both general physical examinations and hospitalization. But what is biochemical test? Who should have biochemical test? What are the items included in the biochemical test? What should we pay attention to before doing it? Today, we will give you a clear explanation in one article!
2021-11-10
1. The blood sample should be transferred into the anticoagulation tube as soon as possible after collection and inverted up and down 3-5 times. This is to ensure that the sample and anticoagulant are well mixed. 2. Whole blood must be analyzed or converted to plasma and serum within 30 minutes of collection. If the test is performed after the sample has been out of the body for a longer period of time, the accuracy of the test results may be affected.