release time:2021-09-03 17:04:16
Seamaty fully automatic dry chemistry analyzer uses absorption spectroscopy colorimetric method and transmission turbidimetric method. This dry chemistry analyzer tests specimens such as lithium heparin anticoagulated whole blood, plasma or serum. The sample size is only 90-120 ul. The test time is only 12 minutes per sample.
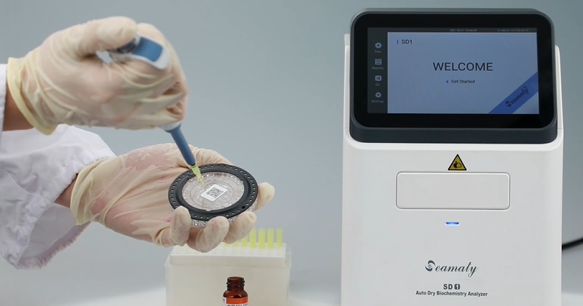
Seamaty dry chemistry analyzer test results can be automatically printed and stored. SD1 truly automates testing. SD1 dry chemistry analyzer is small, easy to operate, stable instrument performance, high precision and easy to carry. It is especially suitable for use in hospital outpatient clinics, emergency clinics, maternal and child medical examinations, mobile blood collection vehicles, blood collection houses, primary medical service institutions and families.
Seamaty is a fast growing medical
device manufacturer specializing in the development, manufacture and marketing
of clinical diagnostic products.
Based in Chengdu, China, Seamaty
manufactures a wide range of IVD devices and reagents based on blood tests,
biochemical diagnostics, immunodiagnostics, and POCT.
Seamaty clinical test analyzers are
supported worldwide by a qualified business development and service support
team. We provide support in other countries through highly trained and
professional distribution partners.

2025-03-18
Catch the pulse of healthcare efficiency with an exploration of why automated blood cell counters are indispensable for clinical labs. Dive into Seamaty's SMT-30 and SMT-50 hematology analyzers, unveiling a world of precise diagnostics and enhanced productivity.
2021-11-15
As the standard of living improves, the number of dogs and cats with obesity and advanced age is increasing. Diabetes has also become the number one chronic disease plaguing pets. Some data show that about 1 in 100 dogs have the worry of diabetes. And the worrying thing is: this number is still on the rise.

2021-10-22
The following is an introduction to the process of CE certification for medical devices.Step 1: Determine and analyze the exported device. Determine whether your medical device is within the scope of the 3 medical device directives of the EU.