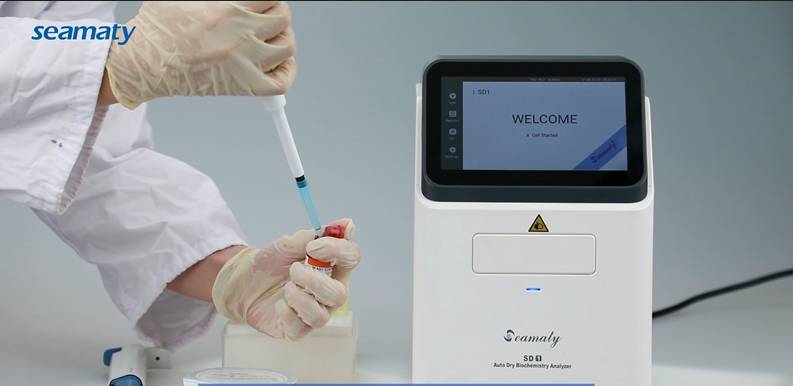
In Vitro Diagnosis refers to products and services that are used outside the human body to obtain clinical diagnostic information by testing human samples (blood, body fluids, tissues, etc.) to determine diseases or body functions.
The in vitro diagnostic industry is the "tool" and "weapon" of laboratory medicine. At the same time, laboratory medicine is the "user" and "market" of the in vitro diagnostic industry. The common purpose of both is to perform in vitro diagnostics. About 80% of clinical diagnostic information comes from in vitro diagnostics. And its cost is less than 20% of the medical cost. In vitro diagnostics has become an increasingly important part of human disease prevention, diagnosis and treatment.
In vitro diagnostic products are mainly composed of diagnostic equipment (instruments) and diagnostic reagents.
Types of in vitro diagnostic instruments
In vitro diagnostic instruments if classified according to the way of matching reagents. Instruments can be divided into open system and closed system.
Types of in vitro diagnostic reagents
The types of in vitro diagnostic reagents are biochemical diagnostic reagents, immune diagnostic reagents, molecular diagnostic reagents, microbial diagnostic reagents, urine diagnostic reagents, coagulation type diagnostic reagents, hematology and flow, cellular diagnostic reagents.
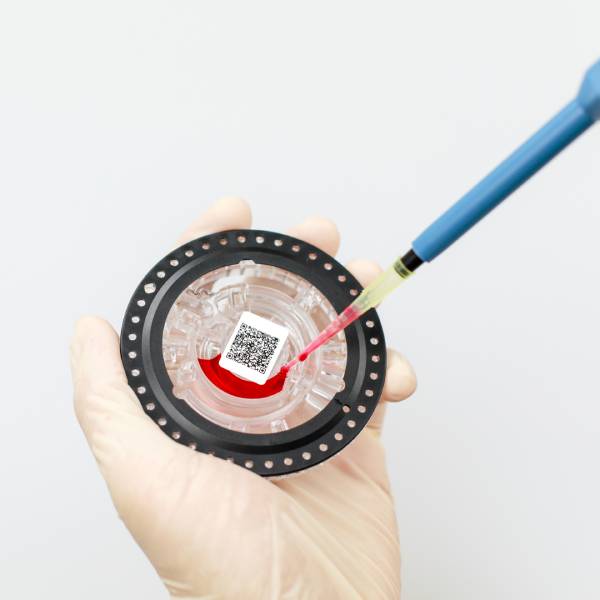
What devices are included in in vitro diagnostics? What polymer materials are used in these devices?
In vitro diagnostics are mainly concentrated in 3 major areas: biochemical diagnostic reagents market, immunological diagnostic reagents market and hematology and flow cytometry market. Commonly used equipment includes blood cell analyzers, microbiological tests, biochemical analyzers, etc.
There are about a dozen kinds of medical plastic materials in common use, including polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polyurethane (PU), polytetrafluoroethylene (PTFE), polycarbonate (PC), polystyrene (PS), etc. Among them, PVC and PE account for the largest amount, 28% and 24% respectively; PS accounts for 18%; PP accounts for 16%; engineering plastics account for 14%.