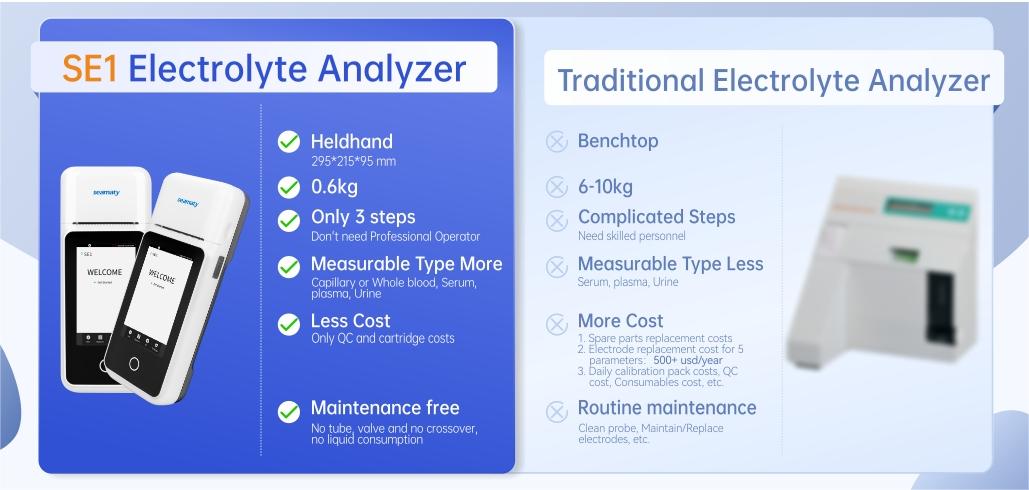
In vitro diagnosis and its miniaturization
In vitro diagnosis (IVD), as the name implies, refers to the products or services that test human blood, body fluids, tissues, etc. to obtain clinical information.With the development of technology and in order to meet different diagnostic needs, in vitro diagnosis is gradually developing into two levels. The robotic, automated, assembly-line central laboratory and the simple, fast and portable
point-of-care testing (POCT).
For large hospital testing departments or third-party testing centers, due to the large volume of samples, automated assembly-line central laboratories are often better able to meet their needs for more samples per unit of time and lower costs for individual sample testing. Point-of-care testing, known internationally as POCT, is the immediate analysis of samples at the sampling site. This eliminates the time required to transfer specimens to the testing laboratory. This is also a new class of methods to quickly obtain test results. Such tests usually do not necessarily require professional staff such as clinical laboratory technicians to carry out. Compared to traditional laboratory testing mechanisms, POCT is a portable, in situ test that is partially completed by non-professionals, more accessible and adaptable, mainly through streamlined operational processes, integrated testing devices, and compressed testing costs.
Because of its rapid and simple advantages, POCT is important for clinical departments such as emergency departments and intensive care units (ICUs) in hospitals. Because of its comprehensive cost, portability and ease of use, POCT is also important for improving medical construction in remote rural areas, accelerating the inspection and quarantine process, responding to unexpected disease disasters, and promoting personalized medicine and disease screening, etc. POCT is now widely used in clinical treatment and monitoring.
Problems in immediate diagnostic (POCT) equipment
The most common POCT devices we use are immunochromatographic test strips like early pregnancy test strips. The immunochromatographic reaction is actually the directional diffusion of the liquid sample on the test paper from one end of the paper to the other. During the directional diffusion of the liquid sample there is a biochemical reaction with the reagents preloaded on the test paper. The final result is visible to the naked eye on the test paper. Because of its simplicity, convenience and speed, it has been used until now.
However, it is difficult to achieve uniform structure of this test paper. Material, thickness, sparseness is also difficult to be identical, which will make the sample test results on different test cards consistency is poor. In addition, the uncontrollable flow speed, sample volume, and reaction time of the sample under test will further increase the bias of the sample detection results. In addition, immunochromatographic test strips are limited by their own methodology and can generally only achieve qualitative diagnosis and cannot meet the quantitative needs of physicians. Finally, for immunochromatography, the biggest problem still lies in relying on the unidirectional diffusion of the liquid on the paper, and the space in which the liquid can be manipulated is also greatly limited. Therefore, many clinically very good diagnostic techniques cannot be achieved by immunochromatography because of the relative complexity of the operation.
Microfluidics
Microfluidics is a technology that relies on modern microfabrication processes to handle or manipulate microfluids at the micron size level (typically one to several hundred microns). This technology enables the integration of experimental processes such as sampling, dilution, reagent addition, separation, detection, and various biochemical reactions on chips of a few square centimeters or smaller. This allows for reduced sample reagent consumption, improved detection sensitivity, shorter reaction times, and lower average costs. At its core is a highly integrated and miniaturized microfluidic chip. That is why microfluidics is also often referred to as Lab on a Chip (LOC). Microfluidics can meet the needs of different scales of object detection, from small biological molecules to cells, and by coupling optical, electrical, thermal and other forms of detectors and readout devices at the back end. This allows automation of the detection process and informatization of the test results.
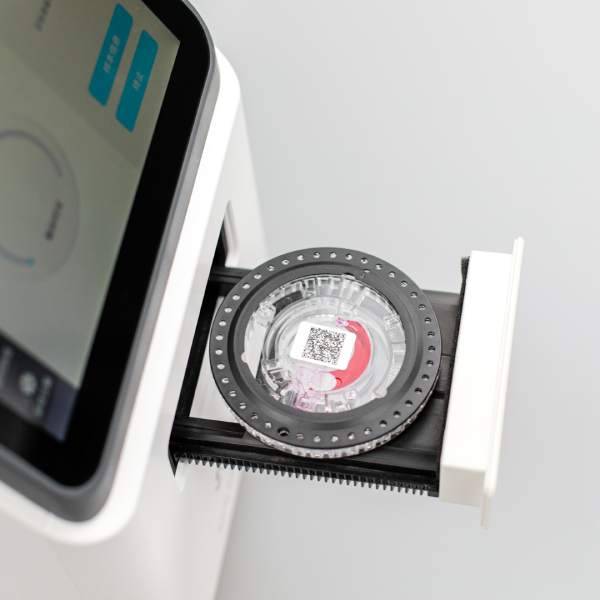
The miniaturization, integration and automation of diagnostic instruments based on microfluidics are highly relevant to the development of POCT and are of great significance to optimize clinical testing. It has increasingly become a research hotspot and core technology in the field of POCT in recent years.
Application of microfluidic technology in POCT
In vitro diagnostics mainly consists of three major branches: biochemical analysis, immunodiagnosis and molecular diagnosis.
In the field of biochemical analysis and immunodiagnosis, where the automation technology is relatively mature. Due to the high integration of microfluidic chips, microfluidic instruments often have the advantages of easy operation, relatively inexpensive instruments, small and light, and can produce results quickly. However, due to the more mature technology of traditional robotic testing instruments, more samples can be tested per unit of time. Therefore, products based on microfluidics are also facing strong competitive pressure from traditional instruments. However, with the popularity of graded diagnosis, the advantages of microfluidic products with low cost, easy operation and fast results for primary community hospitals have gradually come to the fore.
For biochemical analysis, in addition to conventional diagnosis, microfluidic technology can also bring it into a broader application area. As we know, wearable or other home medical monitoring devices can only initially monitor heartbeat, walking steps, blood pressure and other indicators. If we use microfluidic technology on this basis to achieve simple operation, portable, low-cost home urine, blood and other related components monitoring equipment. Then the data collected will be combined with big data, which will better achieve human health management and thus reduce the cost of chronic disease management. In addition, the emergence of wearable devices based on microfluidic technology for real-time monitoring of sweat components also injects fresh blood into the current relatively depressed wearable device market.
In the diagnosis of pathogenic infectious diseases, molecular diagnosis can significantly shorten the diagnostic "window period" and give accurate quantitative results. So it is often a "gold standard". For example, we are trying to diagnose whether a patient is infected with the hepatitis B virus. If we use immunodiagnosis, we have to wait until the patient's own immune system recognizes the hepatitis B virus and produces sufficient amounts of hepatitis B antigen or antibody so that we can determine whether the patient is infected with the hepatitis B virus by the presence of hepatitis B antigen or antibody in the blood. The molecular diagnostic technique is much more straightforward. In the early stages of infection, we can determine whether a patient has the hepatitis B virus by detecting the presence of a specific target gene in the patient's blood, and thus identify whether the patient is infected. We can even determine the amount of hepatitis B virus in a patient's blood by precisely measuring the amount of the target gene in the patient's blood. This indicator is particularly critical in the follow-up treatment.
In addition to the incomparable advantage of immunodiagnosis in pathogenic infectious diseases, molecular diagnostics also plays an important role in the diagnosis and treatment of genetic diseases and tumors. Therefore, in recent years, molecular diagnostics has become one of the fastest growing and most promising branches in the field of in vitro diagnostics.
Although the advantages of molecular diagnosis technology are obvious, molecular diagnosis is also expensive due to its tedious steps, time-consuming process, the need for professional personnel, and the high cost of setting up clinical molecular diagnosis laboratories in general. At the same time, due to the complicated sample pre-processing process in molecular diagnostics, precise temperature cycle control is often required as well. This has led to the traditional robotic approach has not been able to automate the whole process well.
The emergence of microfluidics has solved these problems. Microfluidics integrates cell lysis, nucleic acid extraction and amplification, and final detection on a single chip. The reaction process is in a closed environment, reducing the burden on the operator and the possibility of contamination. The micro-controlled flow realizes the need for rapid testing anytime, anywhere, and brings great help to medical testing and disease prevention and control. The two most typical products here are Cepheid's GeneXpert PCR analyzer and BioFire's FilmArray™ multiplex PCR system. Due to the success of these two products, here Cepheid was acquired by Danaher in September 2016 for $4 billion. BioFire was announced to be acquired by BioMerieux in September 2013 for $450 million. And in recent years, molecular diagnostics has begun to fully benefit from microfluidics with significant improvements in sample preparation, which has led to a strong penetration of microfluidics into the Point of Care Testing space since then.
Microfluidics is automating, miniaturizing, and POCTing molecular diagnostics, which will also change the way people are currently treated. In the case of the most common human disease, the cold, for example, currently doctors generally administer drugs based on their clinical experience. In contrast, filmArray can use the nasopharyngeal secretion collected from nasopharyngeal swabs to accurately determine the type of bacteria or virus causing a cold within an hour or so, and can also determine whether the corresponding pathogen has common drug resistance genes, thus enabling accurate drug administration. Molecular diagnosis based on microfluidic technology is of course not only limited to infectious diseases of the upper respiratory tract such as colds, but also introduced microfluidic chips for gastrointestinal tract infections, bloodstream infections, sexually transmitted infections, and HPV typing on top of the common chip-driven, nucleic acid detection platform. The emergence of such products has not only significantly improved medical standards, but also effectively avoided the global problem of bacterial resistance caused by the abuse of broad-spectrum antibiotics.
Problems in the industrialization of microfluidic technology
The industrialization of microfluidics has been facing the problem of immature industry chain leading to high cost of product development and production.
A mature microfluidic product often requires supporting reagents, core microfluidic chips, chip drive platforms, photoelectric detection modules, signal processing modules, and human-machine interaction software systems and other components. For a mature industry chain, different components of a complex product are mass-produced by different companies, and then assembled by a company that has one or more core technologies. The most typical representative here is the smartphone. Even a company as well-funded as Apple cannot have all the components such as CPU, memory, screen, etc. in its hands. But in the industrialization of microfluidics, because the technology is not too mature, the product lacks the corresponding standardization and standardization, it is not yet possible to realize the universalization of components. This makes it impossible to form a cooperative model of developing a product with upstream and downstream companies. The microfluidic products themselves are a combination of microcomputer electrical processing, life sciences, chemical synthesis, optical engineering and electronic engineering and many other fields of discipline of new products, high technical requirements, the development cycle is long. This also makes it difficult to achieve real profitability for breakthrough products such as the GeneXpert PCR analyzer.