Medical equipment is a high-risk special product directly related to personal health and safety. How to scientifically evaluate and formulate the use period of medical equipment, not only to ensure the safety and effectiveness of the product within the expiration date, but also to maximize the use of the product and improve the utilization rate, is a real problem in front of medical equipment manufacturers.
As a common medical device, in vitro diagnostic equipment is usually used to measure various human biochemical indicators.
In vitro diagnostic(IVD) equipment
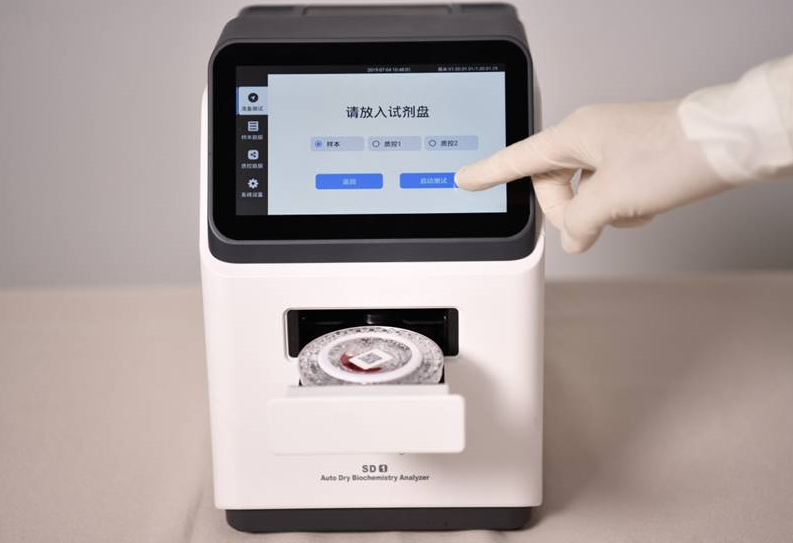
In vitro diagnostic equipment is usually used to test human samples (such as blood, body fluids, tissues, etc.) to obtain clinical diagnostic information and then judge diseases or body functions. Many fully automated in vitro diagnostic devices completely imitate and replace manual operations. For example, fully automated biochemical analyzers not only improve work efficiency, but also reduce testing errors and improve the accuracy and precision of test results.
The system composition of a typical in vitro diagnostic device is usually composed of optical system, mechanical motion system, liquid circuit system, electronic control system and software system in terms of structure. It can be seen that in vitro diagnostic equipment is a more complex system in terms of product structure, involving multiple fields such as mechanical, optoelectronic, microelectronic and communication, which inevitably leads to the complexity of analyzing the service life of related equipment.
The main factors affecting the service life of in vitro diagnostic equipment
Determining the service life of active medical devices mainly considers the following influencing factors, the life of key components, frequency and intensity of use, transportation storage and use environment, the impact of cleaning, disinfection and sterilization, maintenance and repair of components, empirical data and commercial factors, etc. The following is an analysis of the factors influencing the service life of in vitro diagnostic devices.
1) Key components
For in vitro diagnostic equipment, the basic structure and functional modules are clarified through the analysis of typical products. According to the functional modules, it mainly includes sample feeding module, control module, detection module and data processing module, etc. The analysis according to the structure mainly includes mechanical part, optical part, circuit part, software, etc.
Determining the service life of the main components can be done by accelerated aging, fatigue testing, simulation modeling, etc. The evaluation methods that can be used for different structures.
When evaluating key components, in addition to considering the degradation of the safety and effective performance of key components over time, it is also necessary to consider whether there is an interaction between these components that leads to the degradation of the expected safety and effective performance of the components. Also consider the impact of the production and manufacturing process on the life of the component.
2)Use frequency and strength
Under the same environmental conditions, the impact of frequency and intensity of use on the life of the device is inevitable. As mentioned earlier, in vitro diagnostic devices are commonly used in medical institutions. Their average daily continuous use time is about 6h or more, and the frequency of use is high. This is the key factor affecting the service life of the instruments.
3) Transportation, storage and use environment
The expected use environment of in vitro diagnostic equipment is usually in the fixed laboratory of medical institutions. The laboratory environment is relatively stable in terms of temperature, humidity and air pressure. The transportation, storage and use environment of this type of equipment, its life is not greatly affected by the climatic environment and mechanical conditions.
4) Cleaning, disinfection and sterilization
The in vitro diagnostic equipment does not involve cleaning, disinfection and sterilization. Some parts such as sampling parts, reaction parts, cleaning parts, stirring parts, etc. need to be inspected and cleaned on a daily basis. The cleaning method is usually simple and does not involve corrosive materials or methods that affect the instrument's service life. However, the frequency of cleaning is high, and it may be necessary to clean every measurement. Therefore, the impact of cleaning on the service life needs to be considered.
5)Maintenance and repair situation
Generally speaking, medical equipment is usually maintained by the manufacturer's designated maintenance personnel, and users are not allowed to disassemble, modify, or disassemble the product without permission. Because this can avoid the risk of maintenance and repair of components in terms of personnel.
The above 5 are the factors that mainly affect the service life of in vitro diagnostic equipment.