In vitro diagnostics refers to products and services that determine diseases or body functions by testing human samples (blood, body fluids, tissues, etc.) outside the human body to obtain clinical diagnostic information. In vitro diagnostics mainly includes molecular diagnostics, immunodiagnostics, in vitro biochemical diagnostics, etc. At present, in vitro diagnosis is the largest application scenario of microfluidic technology. In vitro diagnostic technology mainly shows the development trend of automation, rapidity, ultra-high sensitivity, high-throughput detection and non-invasive and minimally invasive.
The following section describes in detail from molecular diagnosis, immunodiagnosis, and in vitro biochemical diagnosis, respectively.

1. Molecular Diagnosis
1)Definition of molecular diagnosis
Molecular diagnosis is a diagnostic method to detect nucleic acid and protein changes of individual patients or exogenous pathogens carried by them at the molecular level to assess the health status of patients. Molecular is widely used in infectious disease and genetic disease detection, early tumor screening, prognosis assessment and medication guidance, non-invasive prenatal diagnosis and other fields. Microfluidic microarray technology has been used more in the field of molecular diagnostics. In this paper we mainly go through microfluidic PCR chip in detail.
2) PCR
PCR (Polymerase chain reaction), is a molecular biology technique that can be used to amplify specific DNA fragments. The method can be performed in vitro in organisms. the PCR process consists of 3 steps: denaturation, annealing, and extension. It needs to be performed at 3 different temperatures (high temperature denaturation: 90°C to 95°C, low temperature annealing: 55°C to 60°C, and medium temperature extension: 70°C to 72°C) in high and low.
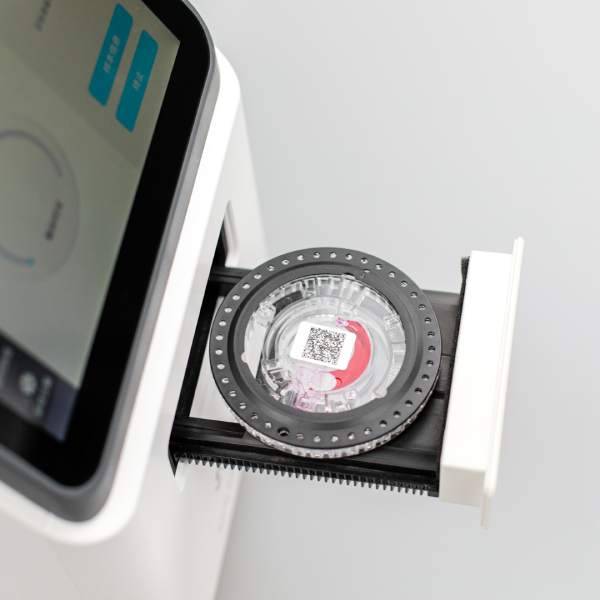
3) Microfluidic PCR chip device and principle
Microfluidic PCR chip is a PCR reaction device based on microfluidic technology. The device is shown in the figure below. The microfluidic device has the advantages of small size, small sample requirement, low cost, full containment, high integration, and fast temperature change, which is beneficial to significantly improve the amplification speed and avoid sample contamination.
Among them, PCR chips are designed for temperature control in a variety of ways.
Spatial type (fluid movement in microchannels with fixed temperature regions): snake form, radiation, closed loop
Temporal type (fluid does not move in the microchannel, temperature changes within the channel): centrifugal form
LAMP chip (constant temperature amplification, no temperature change during amplification)
Digital PCR: samples are diluted to the single molecule level and distributed equally among tens to tens of thousands of units for the reaction. The fluorescence signal of each reaction unit is collected at the end of amplification. Finally, the original concentration or content of the sample is calculated by direct counting or Poisson distribution formula.
4) Advantages of microfluidic PCR chip
Microfluidic PCR technology can effectively reduce the reaction system and improve the reaction efficiency. Microfluidic PCR technology and the integration and miniaturization of different experimental processes. It changes the traditional PCR technology has a long reaction time, high energy consumption, not easy to integrate and portable and other defects.
2. Immunodiagnosis
1) Overview
Immunodiagnosis, as the name implies, is the application of immunological theories, techniques and methods to diagnose various diseases and determine immune status. In medicine, it is an important method to determine the cause and site of disease or to determine whether the immune status of the body is normal.
2)Direction of diagnosis
According to the needs of different diseases, many companies have developed various microfluidic immuno-chips with qualitative or quantitative detection. The diagnostic directions can be divided into: tumor marker detection, antigen and antibody detection for infectious diseases, autoantibody detection and hormone detection, etc.
Tumor Marker Assays
Tumor-specific biomarker levels can be measured to provide information about the disease at an early stage. It can also be used to monitor the effect of anti-tumor drug therapy.
Infectious disease antigen and antibody testing
Some infectious diseases caused by pathogens are highly contagious. Therefore, the ideal test should be immediate, so that the patient can be diagnosed and treated at the site of the test. A number of microfluidic microarrays have been successfully used to identify molecular markers of pathogens and diagnose infections.
Autoantibody testing
Autoantibodies can be found in most autoimmune diseases. Autoantibody concentrations increase in the early stages of disease or in the pre-disease period. Thus, autoantibodies have early warning value. Currently, many autoantibodies are used in routine clinical tests for autoimmune diseases. This is of great value to the diagnosis, treatment monitoring and prognosis of autoimmune diseases.
3. In vitro biochemical diagnosis
1) In vitro diagnosis - biochemical diagnosis
Clinical biochemical analysis is a method based on spectrophotometric testing of dozens of biochemical indicators such as blood glucose and blood lipids, liver function, kidney function and cardiac enzyme profile in blood, urine and other body fluids. Biochemical analysis is one of the most commonly used methods for clinical in vitro diagnosis. Biochemical tests account for nearly 30% of the in vitro diagnostic market.
However, the biochemical tests commonly used in medical institutions were basically done by large automated biochemical analyzers in the past. Although these devices have high throughput, the time taken for a single sample to be sampled to produce a result is long. Such large biochemical analyzers have a high consumption of samples. And because of the cost of analyzer operation on the number of samples required. This does not meet the need for rapid on-site testing and contradicts the need for prevention and control of many chronic diseases that require long-term monitoring.
2) Advantages
-
Fully integrated automation: Microfluidic biochemical analysis system uses microfluidic chip technology to integrate serum blood cell separation, serum quantification, serum dilution and distribution required for blood biochemical analysis on a plastic chip.
-
Rapid Testing: Combined with the portable assay device, it can realize the biochemical assay of a drop of blood in a short time. The portable biochemistry analyzer is significantly less time-consuming and sample-consuming than traditional methods.
-
Simple operation: All reagents are stored on the chip, making it easy for the user to operate.