release time:2021-09-09 09:42:39
Hospital necessary medical apparatus in accordance with the various levels have different requirements. General hospital medical equipment is divided into three categories.

Class I
Through the routine management of medical devices sufficient to ensure their safety and effectiveness.
The safety and effectiveness of medical devices should be controlled.
Implanted in the human body; used to support, sustain life; potentially dangerous to the human body, the safety and effectiveness of medical devices must be strictly controlled.
Seamaty is a fast growing medical device manufacturer specializing in the development, manufacture and marketing of clinical diagnostic products.
Based in Chengdu, China, Seamaty manufactures a wide range of IVD devices and reagents based on blood tests, biochemical diagnostics, immunodiagnostics, and POCT.
Seamaty clinical test analyzers are supported worldwide
by a qualified business development and service support team. We provide
support in other countries through highly trained and professional distribution
partners.
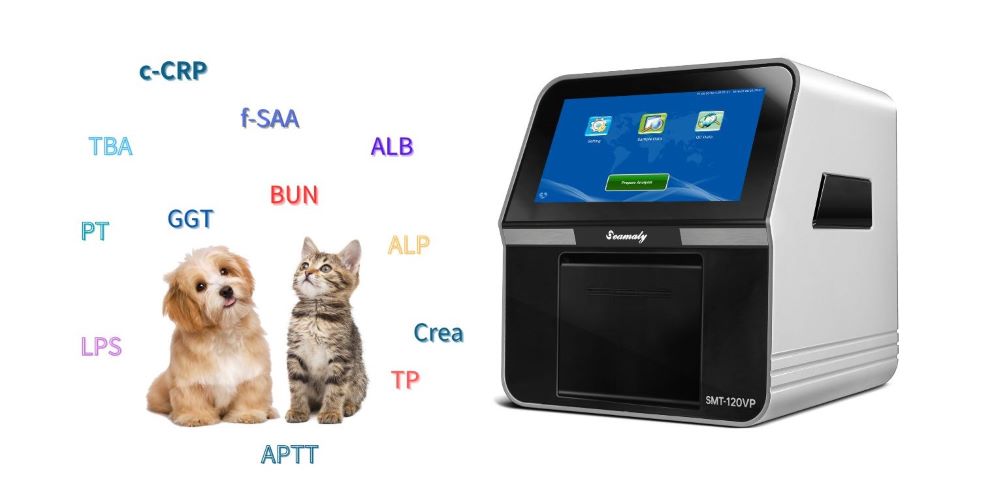
2024-05-21
Unsure what tests POCT veterinary biochemistry analyzers can perform? This comprehensive guide explores the wide range of parameters these analyzers can measure, including kidney function, liver function, blood gases & electrolytes, inflammation, diabetes, and coagulation.
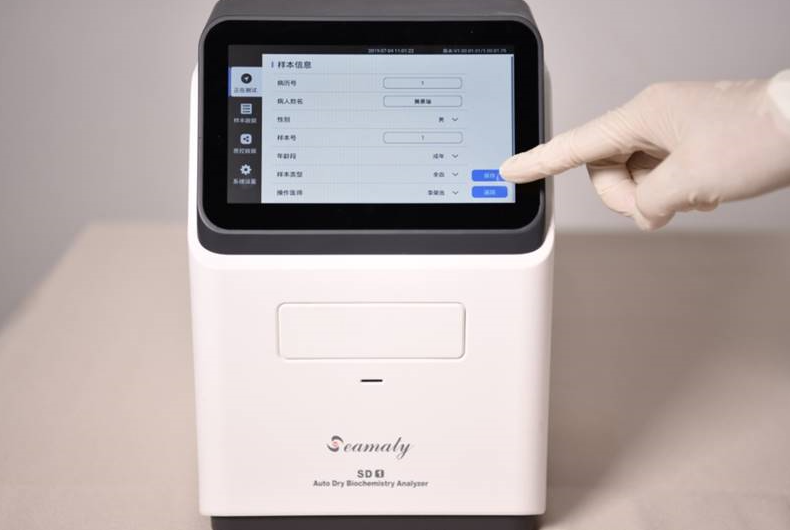
2022-07-04
In a clinical laboratory, a chemistry analyzer is an instrument that is used to measure concentrations of various biomolecules in body fluids. The results of these measurements can provide important information about a patient's health.
2022-03-30
In clinical practice, blood biochemical indicators such as creatinine (Crea), urea (Urea) and uric acid (UA) are commonly used to determine the quality of kidney function. Today, let's get to know more about creatinine.