release time:2025-01-06 09:37:03
In vitro diagnostics (IVD) involves testing human blood, body fluids, or tissues to obtain clinical information. As technology advances, IVD has evolved into two distinct directions:
POCT devices are crucial for clinical settings like emergency rooms and ICUs due to their speed and convenience. Beyond hospitals, POCT contributes to healthcare in remote areas, disaster response, personalized medicine, and disease screening.
The most common POCT devices are immunochromatographic test strips, such as pregnancy test kits. These devices operate by allowing liquid samples to diffuse through a test strip, triggering a biochemical reaction with preloaded reagents. Results are visible to the naked eye.
However, traditional POCT devices face significant limitations:
What is Microfluidics?
Microfluidics involves manipulating micro-scale fluids using advanced fabrication techniques. It integrates complex biochemical processes—such as sampling, reagent mixing, and detection—into a single microchip, often referred to as a "Lab on a Chip" (LOC).
Key benefits of microfluidic technology include:
Microfluidics has become a cornerstone of modern POCT, enabling the development of miniaturized, integrated, and automated diagnostic instruments.
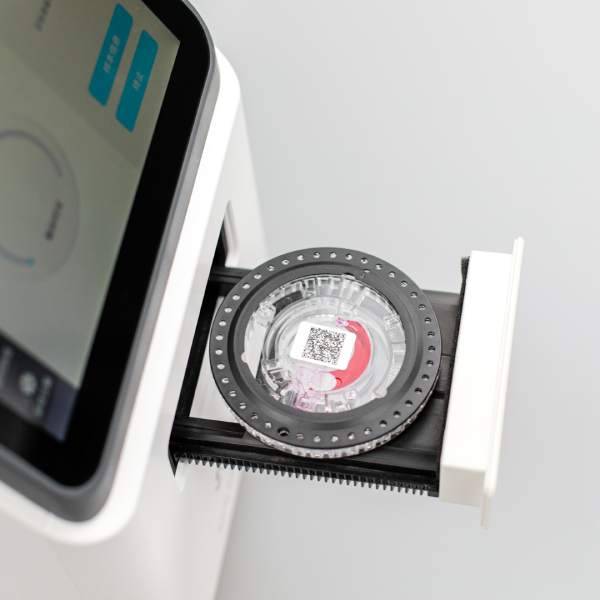
Applications of Microfluidics in POCT
In biochemical and immunodiagnostic testing, microfluidic devices offer easy operation, portability, and rapid results. While traditional robotic systems handle large volumes more efficiently, microfluidic POCT devices excel in cost-effectiveness and accessibility, particularly in primary care settings and community hospitals.
Beyond conventional applications, microfluidics extends its utility to wearable and home diagnostic devices. For example, microfluidic chips can enable portable urine or blood testing, integrating results with big data systems for chronic disease management. Wearable devices using sweat analysis provide novel opportunities for continuous health monitoring.
Molecular diagnostics is often considered the "gold standard" for detecting infectious diseases, genetic conditions, and cancers due to its precision and ability to deliver quantitative results. However, traditional methods are costly, time-consuming, and labor-intensive.
Microfluidics addresses these challenges by integrating complex processes—such as cell lysis, nucleic acid extraction, and amplification—into a single chip. Benefits include:
Notable examples include:
These advancements not only improve diagnostic accuracy but also enable targeted treatments, such as identifying drug-resistant bacteria. Molecular diagnostics has expanded into various fields, including gastrointestinal infections, bloodstream infections, and HPV typing, driven by microfluidic technology.
The widespread adoption of microfluidics in diagnostics faces industrial barriers:
For example, despite its success, the GeneXpert PCR Analyzer struggled with profitability due to its high production and development costs.
Microfluidics is reshaping the POCT landscape by making diagnostic devices smaller, faster, and more accessible. From clinical labs to wearable devices, its applications are vast and transformative. Overcoming current industrial challenges will pave the way for broader adoption, enabling precise, cost-effective, and patient-centric healthcare solutions.

2022-08-28
You are likely aware of the importance of blood testing for diagnosing and treating patients. One common type of blood test is a hematology analysis, which can help detect various diseases and conditions. A 3-part auto hematology analyzer is one type of machine that can be used for this purpose. Here's what you need to know about them.

2022-08-04
Biochemical analyzers are devices that automate the operation of biochemical analysis processes using physical, chemical, and computer technologies. The automated process of a biochemistry analyzer can include: identification and sample delivery, loading of samples and reagents, charging reaction and detection conditions (e.g., wavelength, temperature, time), data processing, and system maintenance.

2021-08-31
1. Blood cell analyzer: blood cell analysis (blood routine) 2. Semi-automatic / fully automatic biochemical analyzer: biochemical tests (such as: liver function, kidney function, blood glucose and lipids ...) 3. Electrolyte analyzer: electrolyte detection: potassium, sodium, chloride, calcium 4. Blood coagulation instrument:blood coagulation items: PT, APTT, TT, FBI ...