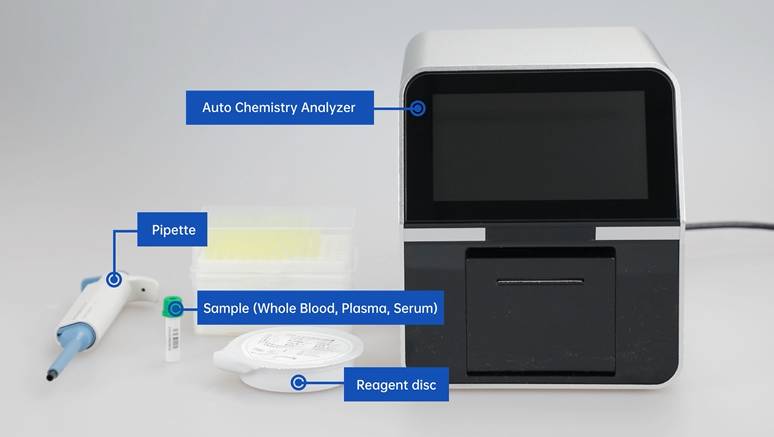
Biochemical reagents have certain requirements on their purity and technology according to their applications. For example, enzyme reagents include crude enzymes, crystalline enzymes, multiple crystalline enzymes and enzyme preparations without certain miscellaneous enzymes.
There are three production methods of biochemical reagents.
-
① separation and purification from organisms.
-
② Chemical synthesis.
-
③Fermentation.
The technical requirements for biochemical reagent products include: content, melting point, freezing point, rotational brightness, water content, spectral characteristics, refraction, density and biological activity, etc.
Types of biochemical reagents
(1) Immunological reagents
Antibodies and antisera, normal serum and complement, antigens, reagents for immunohistochemistry research, reagents for cell culture, cell separation reagents, in-gel diffusion method and electrophoresis reagents, etc.
(2) reagents for genetic engineering
Including gene expression and gene recombination, synthetic proteins, hormones, nucleic acid synthesis reagents, nucleic acid preparations, endonucleases, etc.
(3) mutagens and carcinogens
Mainly for the determination of carcinogenicity of toxic substances in the workplace and living environment and mutagenicity of chemical toxins.
(4) Clinical diagnostic reagents
They are mainly used for clinical pathology diagnosis, biochemical diagnosis, liquid crystal diagnosis, isotope diagnosis and general chemical diagnosis in the medical system, a large class of chemical reagents used in diagnostic tests.
(5) Industrial chemicals
It includes industrial chemicals developed on a trial basis, and there are more than 4,000 kinds of them, and they are still increasing.
Common biochemical reagent production process
Container cleaning - Weighing (key process) - Dissolving, preparation (filtration) - Semi-finished product inspection - Dispensing - Freeze-drying if any (special process) - Printing lot number, labeling, packaging, boxing - Finished product inspection.
Operation procedures and quality control points of biochemical reagents
A. Main raw materials
The main raw materials closely related to product quality include various enzyme preparations, chemical reagents, antigens, antibodies, etc.
The routine inspection items of the main raw materials generally include:
-
1. appearance
-
2. Content (chemical reagents): according to national and international standards or the internal standards of the enterprise.
-
3. Enzyme activity (enzyme preparation): according to the general standard or the enterprise's internal standard.
-
4. Antibody potency: detection by multiplicative dilution and antigen reaction (it is recommended to detect the quality of the product on arrival through the preparation of samples).
B. Other
1. Packaging bottle Quality requirements: appearance, sealing, resistance to falling, dissolution, decolorization test, oscillation test.
2. Instructions, packaging box, bottle label and other identification.
C. Key steps and quality control items of reagent kit production
The production of reagents includes the steps of water making, preparation, filtration, lyophilization (lyophilized reagents) and sub-packaging of reagents. It ensures the quality of the product meets the relevant regulations through two quality control processes: semi-finished product inspection and finished product inspection.
1. Water preparation: Use water treatment equipment to prepare process water and connect to each water point through special pipeline.
2. Container cleaning
-
(1) Use water to clean the required containers for preparation.
-
(2) Quality control requirements: clean and stain-free.
-
(3) The cleaning of containers should be carried out in accordance with the validation program; there should be requirements for the cleaning water.
-
(4) If there are requirements for container cleaning after drying, there should be a validation report of the drying process parameters.
3. Weighing
-
(1) weighing before the balance should be adjusted to the level and calibrated. Weighing should be double-checked, there should be a balance zeroing process.
-
(2) balance accuracy should be at least one order of magnitude higher than the minimum accuracy of the weighed items.
4. Preparation
-
(1) should have the requirements of the mixing method: such as the use of a mixer should be the rate and time requirements. Such as manual stirring should have the requirements of the number of stirring turns.
-
(2) the preparation room temperature should generally be controlled at 18 ~ 25 ℃. Preparation, filtration time generally does not exceed 4 hours.
5. Filtration
Choose different filter membranes for filtration according to the protocol of each product. To ensure that the solution is free of impurities and the absorbance of the blank meets the requirements. Routine quality control items: reagent appearance, blank absorbance (completed by subsequent inspection of semi-finished products).
6. Dispensing
-
(1) Reagent distribution according to process requirements. Before, during and at the end of dispensing, the amount of dispensing should be checked. Routine quality control items: confirm the reagent name, batch number, quantity, packing quantity and sealing after packing before packing.
-
(2) There should be the requirement of trial dispensing, or the requirement of first bottle testing.
7. Freeze-drying
-
(1) Use lyophilizer to lyophilize reagents. All kinds of lyophilized reagents need to establish the corresponding lyophilization process, and the important parameters of the lyophilization process are mainly lyophilization time, lyophilization pressure and lyophilization temperature.
-
(2) The appearance of lyophilized products should be presented as loose powder solid, and re-soluble completely within the specified time (generally not more than 60 minutes).
-
(3) freeze-drying process is generally: pre-freezing (cooling) - evacuation - frosting [heating and warming (gradually)
8. Packaging
Put each component of the reagent, certificate of conformity and instructions into the corresponding reagent box. When packing, check the name, batch number, expiration date, loading quantity and specification, check the quantity of each material, and double check before covering the box.
9. Sealing film
Put the assembled reagents on the automatic film sealing machine. Control the sealing time and temperature of the film. Make sure the film is complete and tightly adsorbed with the reagent box.
The above is the common production method and process of biochemical reagents. Seamaty biochemical reagent tray adopts microfluidic technology and precise internal independent channel design to effectively avoid cross contamination and ensure the reagent reaction is not interfered by other substances. The reagent tray adopts a special lyophilization process to completely sublimate the water inside the reagents without destroying the active ingredients. Under refrigeration at 2~8℃, it is valid for up to 1 year.