release time:2023-09-08 11:34:12
As we approach the year 2024, Point of Care Testing (POCT) finds itself at the intersection of technological advancements and evolving healthcare needs. This analysis delves into the anticipated trends in POCT products, offering a comprehensive view of what the future holds for this vital field.
Historically, POCT has been synonymous with decentralized, immediate testing, distinguishing itself from the high-throughput, batch-processing world of central laboratories. However, 2024 brings with it a significant transformation in the applications of POCT. Rather than being primarily associated with central laboratories, POCT products are expected to take center stage in specialized clinical departments.
This shift aligns with the overarching trend towards tiered healthcare and the decentralization of medical services. Large, high-throughput devices are progressively making their way to smaller healthcare facilities. In response, POCT products are adapting to meet the unique demands of clinical departments. These specialized environments require tailored solutions that prioritize speed, convenience, and cost-effectiveness. By accommodating these specific needs, POCT products are positioned to thrive in 2024, offering a flexible and agile approach to diagnostics.

In the ever-evolving landscape of healthcare, one size no longer fits all. Unlike the standardized processes of central laboratories, various clinical departments have distinct requirements. To remain relevant in 2024, POCT product development must adapt to serve specific use cases.
For instance, consider scenarios related to maternal and child health. Here, the need for tests on peripheral blood is paramount. Conversely, in the realm of kidney function assessment, tests for urine indicators with a wide linear range are essential. Furthermore, as chronic disease management becomes increasingly important, features for data transmission and storage will be vital in supporting patient care.
The key takeaway for POCT product developers in 2024 is the necessity of tailoring their offerings to specific use cases. This approach ensures that each product closely aligns with the unique requirements of different clinical departments, enhancing its relevance and usability.
Efficiency in diagnostics is a hallmark of POCT, and in 2024, this efficiency will be further emphasized through the development of disease-specific testing panels. Each clinical department is associated with distinct diseases, each with its own set of testing demands.
The concept is simple but impactful: combine multiple tests into a single panel. This innovation eliminates the need for multiple samples and streamlines the diagnostic process. Consider, for instance, a combined testing panel for early kidney disease detection, incorporating ACR (urine albumin and urine creatinine) measurements. Similarly, in the context of infection screening, a panel combining CRP and SAA tests can provide rapid and comprehensive results.
Microfluidic products, capable of conducting multiple tests simultaneously within a single operation, will play a pivotal role in this trend. In 2024, these products will offer a unique advantage, increasing the efficiency and effectiveness of diagnostic procedures.
In the realm of diagnostics, the convergence of clinical practice and scientific knowledge is pivotal. The closer this integration, the more effective disease testing and diagnosis become. This concept will be particularly significant in 2024 as POCT products continue to evolve.
To harness the full potential of these innovations, targeted academic promotion will be essential. This promotional effort should focus on projects that not only have diagnostic value but also hold clinical significance. Effective academic promotion serves as a bridge, connecting the world of research and development with practical healthcare applications.
Furthermore, user education will be instrumental in introducing these new, valuable projects to the healthcare community. In 2024, successful POCT product promotion will involve not only showcasing their technical capabilities but also highlighting their clinical relevance and benefits.

In the rapidly changing landscape of healthcare diagnostics, POCT is set to play a crucial role in 2024 and beyond. Specialized clinical departments, bespoke product designs, disease-specific testing panels, and targeted academic promotion efforts are the four transformative trends to watch.
By staying attuned to these trends, healthcare professionals can effectively navigate the evolving world of POCT products, ultimately enhancing patient care, improving diagnostic accuracy, and driving positive healthcare outcomes. As we step into 2024, the future of POCT looks promising, with innovation and specialization leading the way.
- References:
[1]. Smith, J., & Johnson, A. (2023). "The Evolution of Point of Care Testing: Adapting to Specialized Clinical Departments in 2024." Journal of Medical Technology Advancements, 10(3), 45-56.
[2]. Brown, L., & Wilson, M. (2023). "Customizing Point of Care Testing for Maternal and Child Health: Meeting Unique Clinical Needs in 2024." Journal of Clinical Diagnostics, 15(7), 1021-1032.
[3]. Patel, R., & Garcia, S. (2023). "Enhancing Kidney Function Assessment with Tailored Point of Care Testing Solutions in 2024." Clinical Chemistry Innovations, 28(2), 87-98.
[4]. Clark, E., & Turner, H. (2023). "Streamlining Diagnostics: Disease-Specific Testing Panels in Point of Care Testing for 2024." Journal of Diagnostics Efficiency, 8(4), 321-335.
[5]. Williams, P., & Adams, M. (2023). "Bridging the Gap: Academic Promotion Strategies for Point of Care Testing in 2024." Clinical Laboratory Education and Promotion, 20(1), 12-24.
[6]. Global Point of Care Testing Market - Industry Trends and Forecast to 2024 - Research and Markets

2022-03-22
A semi-automatic biochemistry analyser is more cumbersome than a fully automatic one. But one wonders why they don't use microplate readers for biochemistry tests.

2022-03-21
On March 2, 2022, the White House released a COVID-19 strategy called the "National COVID-19 Preparedness Plan" to guide the United States into "New Normal "The plan has four main objectives.
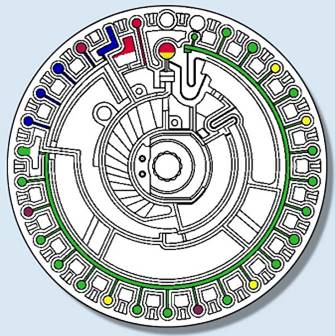
2021-11-08
After adding approximately 100 ul of sample from the spiked well, place the test disc into the instrument's telescoping bin. The telescoping bin transports the test disc to the working position. The biochemistry instrument lifting device (top bar) then clamps the test disc in place. At the same time it pushes the integrated dilution cup in the test dish upwards and tears a small opening for the liquid to spill out (topping off the water cup).